
Chemistry, 07.04.2020 21:50 hunteryolanda82
The Kb of weak base B is 1.0 x 10-6. A solution contains 0.75 M B and 0.25 M HB+Cl- (the salt of B with HCl). What is the pH of the solution after 0.05 mol NaOH is added to 1.0 L of the above solution?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2


Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
The Kb of weak base B is 1.0 x 10-6. A solution contains 0.75 M B and 0.25 M HB+Cl- (the salt of B w...
Questions



Mathematics, 19.07.2019 03:50

Mathematics, 19.07.2019 03:50

English, 19.07.2019 03:50




Mathematics, 19.07.2019 03:50



History, 19.07.2019 03:50


Mathematics, 19.07.2019 03:50


Geography, 19.07.2019 03:50


Biology, 19.07.2019 03:50

Mathematics, 19.07.2019 03:50

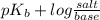
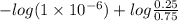
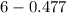
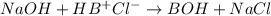
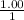 , [Salt] =
, [Salt] = 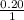
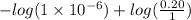 [/tex]
[/tex]



