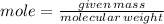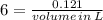To produce 40.0 g of silver chromate, you will need at least 23.4 g of potassium chromate in solution as a reactant. All you have on hand in the stock room is 5 L of a 6.00 M K2CrO4 solution. What volume of the solution is needed to give you the 23.4 g K2CrO4 needed for the reaction?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
To produce 40.0 g of silver chromate, you will need at least 23.4 g of potassium chromate in solutio...
Questions

Advanced Placement (AP), 20.06.2021 04:50



Mathematics, 20.06.2021 04:50

Biology, 20.06.2021 04:50





Arts, 20.06.2021 04:50

Mathematics, 20.06.2021 04:50

Mathematics, 20.06.2021 04:50


Mathematics, 20.06.2021 04:50

Mathematics, 20.06.2021 04:50


Mathematics, 20.06.2021 04:50


Mathematics, 20.06.2021 04:50

 required=20.1 ml
required=20.1 ml