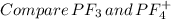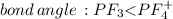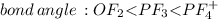Chemistry, 07.04.2020 21:43 ginger1234
Place the following in order of increasing F-A-F bond angle, where A represents the central atom in each molecule. PF3 OF2 PF4
a. PF3 < OF2 < PF4
b. OF2 < PF3 < PF4
c. PF4 < OF2 < PF3
d. PF4 < PF3 < OF2
e. OF2 < PF4 < PF3
f. PF3 < PF4 < OF2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
Place the following in order of increasing F-A-F bond angle, where A represents the central atom in...
Questions

English, 02.09.2019 22:10



Mathematics, 02.09.2019 22:10

Mathematics, 02.09.2019 22:10

Mathematics, 02.09.2019 22:10

History, 02.09.2019 22:10

Mathematics, 02.09.2019 22:10


Mathematics, 02.09.2019 22:10

Mathematics, 02.09.2019 22:10

Mathematics, 02.09.2019 22:10



Social Studies, 02.09.2019 22:10



Social Studies, 02.09.2019 22:10


Computers and Technology, 02.09.2019 22:10

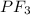 with
with 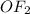
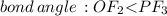
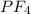 does no exist it exist only in the form of
does no exist it exist only in the form of 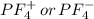 and here assuming
and here assuming 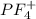 because in case of
because in case of 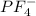 difficult to compare bond angle because here two different type of bond angle is present.
difficult to compare bond angle because here two different type of bond angle is present.