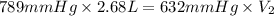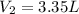Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1
You know the right answer?
You fill a balloon with helium gas to a volume of 2.68L at 23°C and 789mmHg. Then you release the ba...
Questions

History, 08.11.2019 19:31


Mathematics, 08.11.2019 19:31



English, 08.11.2019 19:31

Social Studies, 08.11.2019 19:31



Spanish, 08.11.2019 19:31


Mathematics, 08.11.2019 19:31

Social Studies, 08.11.2019 19:31


Mathematics, 08.11.2019 19:31



History, 08.11.2019 19:31

Mathematics, 08.11.2019 19:31



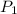 = initial pressure = 789 mmHg
= initial pressure = 789 mmHg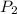 = final pressure = 632 mmHg
= final pressure = 632 mmHg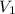 = initial volume = 2.68 L
= initial volume = 2.68 L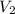 = final volume = ?
= final volume = ?