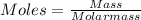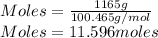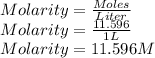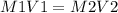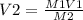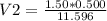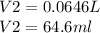Chemistry, 08.04.2020 00:19 rhineharttori
A sample of commercial perchloric acid is 70.0% HClO4 by mass; its density is 1.664 g/mL. How many milliliters of this concentrated HClO4 would be required to prepare 500. mL of 1.50 M HClO4 solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1


Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

You know the right answer?
A sample of commercial perchloric acid is 70.0% HClO4 by mass; its density is 1.664 g/mL. How many m...
Questions


English, 25.05.2021 21:30



English, 25.05.2021 21:30


Mathematics, 25.05.2021 21:30


Mathematics, 25.05.2021 21:30

Mathematics, 25.05.2021 21:30


English, 25.05.2021 21:30

Mathematics, 25.05.2021 21:30

Mathematics, 25.05.2021 21:30


Mathematics, 25.05.2021 21:30