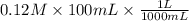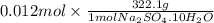Chemistry, 07.04.2020 22:53 99keevintaylor012
The final volume of buffer solution must be 100.00 mL and the final concentration of the weak acid must be 0.100 M. Based on this information, what mass of solid conjugate base should the student weigh out to make the buffer solution with a pH of 2.00?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1


Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
The final volume of buffer solution must be 100.00 mL and the final concentration of the weak acid m...
Questions




Mathematics, 05.02.2021 18:10

Spanish, 05.02.2021 18:10

Biology, 05.02.2021 18:10


English, 05.02.2021 18:10


History, 05.02.2021 18:10

Mathematics, 05.02.2021 18:10

Mathematics, 05.02.2021 18:10



English, 05.02.2021 18:10

Mathematics, 05.02.2021 18:10

Mathematics, 05.02.2021 18:10



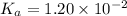
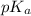 as follows.
as follows.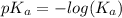
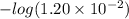
![pK_{a} + log \frac{[A^{-}]}{[HA]}](/tpl/images/0587/7412/24c3e.png)
![1.92 + log \frac{[A^{-}]}{0.1 M}](/tpl/images/0587/7412/4e97a.png)
![[A^{-}]](/tpl/images/0587/7412/fe74d.png) = 0.12 M
= 0.12 M