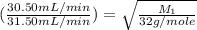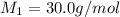Answers: 1
Another question on Chemistry



Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
You know the right answer?
The effusion rate of an unknown gas is measured and found to be 31.50 mL/min. Under identical experi...
Questions

Computers and Technology, 28.01.2020 21:42

World Languages, 28.01.2020 21:42







Mathematics, 28.01.2020 21:42


English, 28.01.2020 21:42









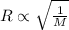
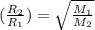 ..........(1)
..........(1)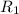 = rate of effusion of unknown gas = 31.50 mL/min
= rate of effusion of unknown gas = 31.50 mL/min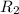 = rate of effusion of
= rate of effusion of 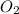 gas = 30.50 mL/min
gas = 30.50 mL/min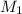 = molar mass of unknown gas = ?
= molar mass of unknown gas = ?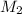 = molar mass of
= molar mass of