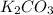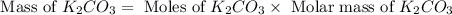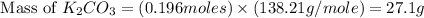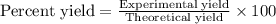Chemistry, 07.04.2020 22:58 timjape3g3z
Determine the theoretical yield and the percent yield if 21.8 g of KCO is produced from reacting 27.9 g KO with 29.0 L of CO (at STP). The molar mass of KO = 71.10 g/mol and KCO = 138.21 g/mol. 4 KO(s) + 2 CO(g) → 2 KCO(s) + 3 O(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

You know the right answer?
Determine the theoretical yield and the percent yield if 21.8 g of KCO is produced from reacting 27....
Questions



Spanish, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50



Mathematics, 18.03.2021 02:50



Computers and Technology, 18.03.2021 02:50

Computers and Technology, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50

Social Studies, 18.03.2021 02:50

World Languages, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50



Mathematics, 18.03.2021 02:50

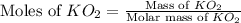
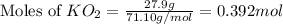
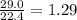 mole of CO₂ gas.
mole of CO₂ gas.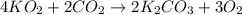
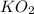 react with 2 mole of
react with 2 mole of 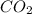
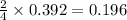 moles of
moles of