
The equilibrium expression for a reaction is keq=[h+]6[bi2+]2[h2s]3. which of the following could be the reaction? a. 6h+(aq) + bis(s) 2bi2+(aq) + 3h2s(g) b. 2bi2+(aq) + 3h2s(aq) bi2s3(s) + 6h+(aq) c. 6h+(aq) + bi2s3(s) 2bi2+(aq) + 3h2s(g) d. 2bi2+(aq) + 3h2s(aq) bi2s3(aq) + 6h+(aq)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3


Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
You know the right answer?
The equilibrium expression for a reaction is keq=[h+]6[bi2+]2[h2s]3. which of the following could be...
Questions



World Languages, 17.01.2022 14:30

English, 17.01.2022 14:30

Mathematics, 17.01.2022 14:30





English, 17.01.2022 14:40

Mathematics, 17.01.2022 14:40

Mathematics, 17.01.2022 14:40

Biology, 17.01.2022 14:40



Mathematics, 17.01.2022 14:40

Biology, 17.01.2022 14:40



English, 17.01.2022 14:50

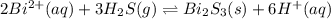
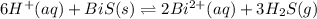
![k_{eq}=\frac{[Bi^{2+}]^2[H_2S]^3}{[H^+]^6}](/tpl/images/0354/4031/90ef6.png)
![k_{eq}=\frac{[H^{+}]^6}{[Bi^{2+}]^2[H_2S]^3}](/tpl/images/0354/4031/d24c1.png)
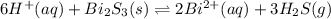
![k_{eq}=\frac{[Bi^{2+}]^2[H_2S]^3}{[H^{+}]^6}](/tpl/images/0354/4031/14e9d.png)
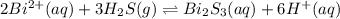
![k_{eq}=\frac{[H^{+}]^6[Bi_2S_3]}{[Bi^{2+}]^2[H_2S]^3}](/tpl/images/0354/4031/e2680.png)



