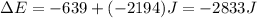Chemistry, 07.04.2020 23:18 tynyiaawrightt
An automobile engine provides 639 Joules of work to push the pistons and generates 2194 Joules of heat that must be carried away by the cooling system. Calculate the change in the internal energy of the engine. E = Joules

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
An automobile engine provides 639 Joules of work to push the pistons and generates 2194 Joules of he...
Questions



Mathematics, 09.03.2021 17:40


Mathematics, 09.03.2021 17:40


Biology, 09.03.2021 17:40



French, 09.03.2021 17:40


Mathematics, 09.03.2021 17:40


Mathematics, 09.03.2021 17:40


Mathematics, 09.03.2021 17:40



History, 09.03.2021 17:40

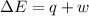
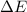 = Change in internal energy
= Change in internal energy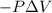 = -639 J {Work is done by the system and is negative as the final volume is greater than initial volume }
= -639 J {Work is done by the system and is negative as the final volume is greater than initial volume }