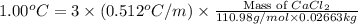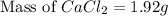Chemistry, 07.04.2020 23:30 mbprez6029
To be able to see a measurable effect, your boiling point elevation must be at least 1.00°C. Knowing that the Kb of water is 0.512\frac{^\circ\text{C}\cdot\text {kg}}{\text{mol}}0.512 ∘ C ⋅ kg mol, determine what mass of calcium chloride (in g) is needed to see at least a 1.00^\circ\text{C}1.00 ∘ C boiling point increase in 26.63 mL of water.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3
You know the right answer?
To be able to see a measurable effect, your boiling point elevation must be at least 1.00°C. Knowing...
Questions



Physics, 21.08.2019 16:30

Mathematics, 21.08.2019 16:30


English, 21.08.2019 16:30

History, 21.08.2019 16:30






English, 21.08.2019 16:30


Biology, 21.08.2019 16:30


Social Studies, 21.08.2019 16:30

Physics, 21.08.2019 16:30


Spanish, 21.08.2019 16:30

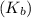 for water =
for water = 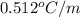
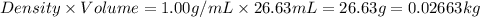
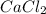 = 110.98 g/mole
= 110.98 g/mole
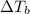 = change in boiling point =
= change in boiling point = 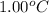
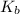 = boiling point constant for water
= boiling point constant for water