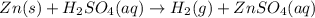Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
Solid zinc metal reacts with sulfuric acid to form hydrogen gas and an aqueous solution of zinc (II)...
Questions






Biology, 20.10.2020 01:01

Mathematics, 20.10.2020 01:01

History, 20.10.2020 01:01


Mathematics, 20.10.2020 01:01


Mathematics, 20.10.2020 01:01

Mathematics, 20.10.2020 01:01



Biology, 20.10.2020 01:01



Mathematics, 20.10.2020 01:01

History, 20.10.2020 01:01