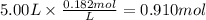The reaction 2N2O5 (g) → 2N2O4 (g) + O2 (g) has a reaction rate that is dependent only on the concentration of N2O5 and at a certain temperature has a rate constant k of 0.0168 s-1. If 2.50 moles of N2O5 were placed in a 5.00 liter container at that temperature, how many moles of N2O5 would remain after 1.00 min?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
The reaction 2N2O5 (g) → 2N2O4 (g) + O2 (g) has a reaction rate that is dependent only on the concen...
Questions




Geography, 02.02.2021 21:30


Geography, 02.02.2021 21:30

Mathematics, 02.02.2021 21:30



SAT, 02.02.2021 21:30







English, 02.02.2021 21:30



Mathematics, 02.02.2021 21:30

![[N_2O_5] = [N_2O_5]_0 \times e^{-k \times t}](/tpl/images/0587/8861/ec7f1.png)
![[N_2O_5]_0](/tpl/images/0587/8861/4a20d.png) : initial concentrationk: rate constantt: time
: initial concentrationk: rate constantt: time![[N_2O_5] = 0.500 M \times e^{-0.0168 s^{-1} \times 60s} = 0.182 M](/tpl/images/0587/8861/ce720.png)