
Chemistry, 07.04.2020 23:53 audrey1256
1. Sulfuric acid is formed when sulfur dioxide gas reacts with oxygen gas and water. Write a balanced chemical equation for the reaction. If 12.5 mol sulfur dioxide reacts, how many mol of sulfuric acid can be produced and how many moles of oxygen gas is needed

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
1. Sulfuric acid is formed when sulfur dioxide gas reacts with oxygen gas and water. Write a balance...
Questions



Biology, 24.05.2021 14:00



Mathematics, 24.05.2021 14:00

Chemistry, 24.05.2021 14:00

Mathematics, 24.05.2021 14:00

Mathematics, 24.05.2021 14:00

Biology, 24.05.2021 14:00

Chemistry, 24.05.2021 14:00

Mathematics, 24.05.2021 14:00

Mathematics, 24.05.2021 14:00

Mathematics, 24.05.2021 14:00

History, 24.05.2021 14:00


Mathematics, 24.05.2021 14:00

Business, 24.05.2021 14:00


English, 24.05.2021 14:00

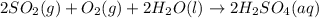
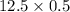 mole of oxygen
mole of oxygen

