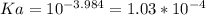Chemistry, 07.04.2020 23:52 brendaesme
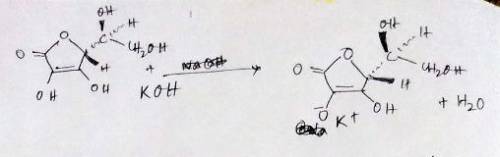
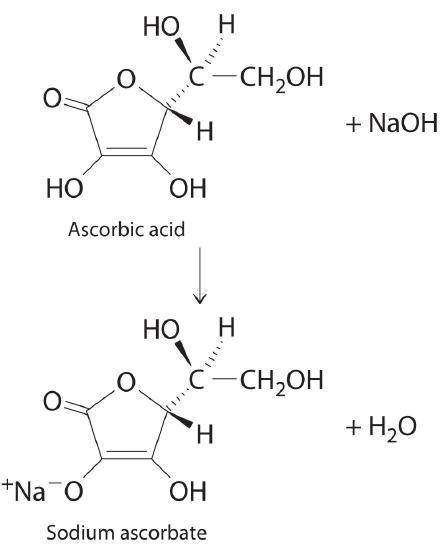
A 0.552-g sample of ascorbic acid was dissolved in water to a total volume of 0.20 mL and titrated with 0.1103 M KOH. The equivalence point occurred at 28.42 mL. The pH of the solution at 10.0mL of added base was 3.72. From this data, determine the molar rmass and Ka for vitamin C.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
A 0.552-g sample of ascorbic acid was dissolved in water to a total volume of 0.20 mL and titrated w...
Questions



Physics, 19.09.2019 02:30

Computers and Technology, 19.09.2019 02:30

Social Studies, 19.09.2019 02:30



Social Studies, 19.09.2019 02:30



Mathematics, 19.09.2019 02:30




Biology, 19.09.2019 02:30



Mathematics, 19.09.2019 02:30

History, 19.09.2019 02:30

Mathematics, 19.09.2019 02:30