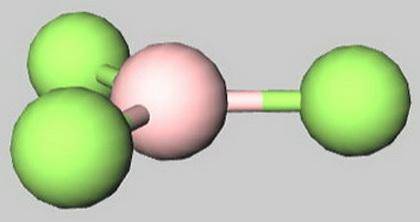
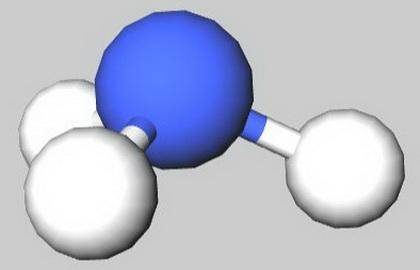
Select the true statements.
A) BF 3 has a trigonal planar shape.
B) Molecules wi...

Chemistry, 07.04.2020 23:39 crystalclear99
Select the true statements.
A) BF 3 has a trigonal planar shape.
B) Molecules with three outer atoms always have a trigonal planar shape.
C) NH 3 has a trigonal planar shape.
D) Trigonal planar molecules have three regions of high-electron density around the central at

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Questions



Engineering, 20.09.2020 02:01

Chemistry, 20.09.2020 02:01


Health, 20.09.2020 02:01



History, 20.09.2020 02:01


History, 20.09.2020 02:01


Social Studies, 20.09.2020 02:01

English, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01

Mathematics, 20.09.2020 02:01