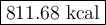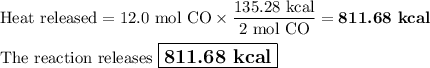Chemistry, 07.04.2020 23:54 hardwick744
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g) → 2 CO2(g) ∆H for this reaction is −135.28 kcal. How much heat would be released if 12.0 moles of carbon monoxide reacted with sufficient oxygen to produce carbon dioxide?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1


Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g)...
Questions

Mathematics, 11.01.2021 01:00

Computers and Technology, 11.01.2021 01:00

SAT, 11.01.2021 01:00

Mathematics, 11.01.2021 01:00

Mathematics, 11.01.2021 01:00

Mathematics, 11.01.2021 01:00



Mathematics, 11.01.2021 01:00


English, 11.01.2021 01:00


Biology, 11.01.2021 01:00

Biology, 11.01.2021 01:00


Mathematics, 11.01.2021 01:00

Law, 11.01.2021 01:00



Mathematics, 11.01.2021 01:10