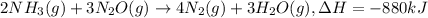When 2 moles of NH3(g) react with N2O(g) to form N2(g) and H2O(g) according to the following equation, 880 kJ of energy are evolved. 2NH3(g) + 3N2O(g)4N2(g) + 3H2O(g) Is this reaction endothermic or exothermic? What is the value of q? kJ An error has been detected in your answer. Che

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3


Chemistry, 23.06.2019 11:30
Distilled water is a completely neutral solution. what is its ph? a. 1 b. 7 c. 14 d. 0
Answers: 2

Chemistry, 23.06.2019 12:00
372 ml is the volume of aluminum, density is 2.70 g/ml what is the mass in grams
Answers: 1
You know the right answer?
When 2 moles of NH3(g) react with N2O(g) to form N2(g) and H2O(g) according to the following equatio...
Questions



English, 22.10.2019 07:00

Mathematics, 22.10.2019 07:00



English, 22.10.2019 07:00

Chemistry, 22.10.2019 07:00

Computers and Technology, 22.10.2019 07:00



Mathematics, 22.10.2019 07:00



Mathematics, 22.10.2019 07:00


Biology, 22.10.2019 07:00

Mathematics, 22.10.2019 07:00

History, 22.10.2019 07:00

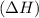 comes out to be negative.
comes out to be negative.