
Chemistry, 07.04.2020 23:59 ryliepeloquinf
A laser is emitting photons with a wavelength of 639.8 nm. What is the energy for 1 mole of these photons? For Planck's constant, use a value of 6.626x10-34 J s. Use units of kJ/mol. Report just the number, not the units.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
A laser is emitting photons with a wavelength of 639.8 nm. What is the energy for 1 mole of these ph...
Questions

Mathematics, 26.02.2021 01:50

Social Studies, 26.02.2021 01:50


Chemistry, 26.02.2021 01:50




English, 26.02.2021 02:00

Mathematics, 26.02.2021 02:00

Mathematics, 26.02.2021 02:00



Mathematics, 26.02.2021 02:00



History, 26.02.2021 02:00

Chemistry, 26.02.2021 02:00

Mathematics, 26.02.2021 02:00


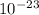
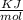
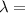 639.8 ×
639.8 × 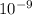 m
m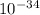 J sec
J sec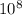 meter per second
meter per second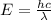
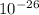
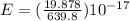
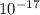 Joule per mole
Joule per mole

