
Chemistry, 08.04.2020 00:56 klmklm3799
Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. The balanced equation for the reaction is: 2Mg(s)+O2(g)→2MgO(s) When 10.1 g of Mg are allowed to react with 10.5 g of O2, 10.3 g of MgO are collected. Dteremine the limiting reactants for the reaction

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:20
Which of the following statements is not true? • a. covalent compounds have low melting and boiling points. • ob. covalent bonds between atoms of a compound are relatively weak compared to bonds between molecules. • c. covalent bonds occur between nonmetals. • d. covalent compounds are often gases or liquids.
Answers: 2

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1


Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. The balanced equat...
Questions

Chemistry, 20.11.2020 01:00




Mathematics, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

History, 20.11.2020 01:00

English, 20.11.2020 01:00

History, 20.11.2020 01:00

Biology, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00


Geography, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Chemistry, 20.11.2020 01:00



Social Studies, 20.11.2020 01:00

Chemistry, 20.11.2020 01:00

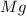 = 10.1 g
= 10.1 g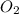 = 10.5 g
= 10.5 g = 32 g/mol
= 32 g/mol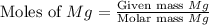
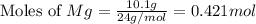
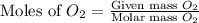
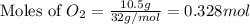
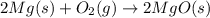
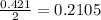 moles of
moles of 

