
Chemistry, 08.04.2020 00:03 landanwithers
Calculate the maximum solubility of silver carbonate, Ag2CO3 in g/L when in the presence of 0.057 M AgNO3. The solubility product of Ag2CO3 is 8.1x10-12 and Ag2CO3 has a molar mass of 167.91 g/mol. Express your answer to the correct number of significant figures, in scientific notation and include units with your answer.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1

Chemistry, 23.06.2019 08:50
What happens after sound waves create vibrations in the ear?
Answers: 1

You know the right answer?
Calculate the maximum solubility of silver carbonate, Ag2CO3 in g/L when in the presence of 0.057 M...
Questions

Spanish, 23.08.2019 16:00

Social Studies, 23.08.2019 16:00








Mathematics, 23.08.2019 16:00

Mathematics, 23.08.2019 16:00

Biology, 23.08.2019 16:00

Mathematics, 23.08.2019 16:00






Social Studies, 23.08.2019 16:00

Mathematics, 23.08.2019 16:00

 .
. 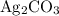 at equilibrium. The solubility equilibrium for
at equilibrium. The solubility equilibrium for 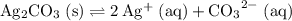 .
. is
is 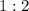 . For
. For  concentration be
concentration be  . The increase in
. The increase in  . Note, that because of the
. Note, that because of the  of
of 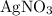 , the concentration of
, the concentration of  .The concentration of
.The concentration of  .
.![\begin{aligned}&\rm \left[Ag^{+}\right]^2 \cdot \left[{CO_3}^{2-}\right] = K_{\text{sp}} \\ & \implies (0.057 + x)^2\cdot x = 8.1 \times 10^{-12} \end{aligned}](/tpl/images/0588/0899/128cb.png) .
. is considerably small. Therefore, at equilibrium, the concentration of
is considerably small. Therefore, at equilibrium, the concentration of  :
: .
. .
. , meaning that there are approximately
, meaning that there are approximately  of
of  .
.

