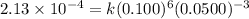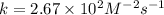Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1

Chemistry, 23.06.2019 12:30
What are some examples of anthropogenic atmospheric particulates?
Answers: 1

Chemistry, 23.06.2019 19:30
⁉️how many kj of energy would be needed to convert 150. g of ammonia to vapor at its boiling point? ⁉️(ammonia’s heat of vaporization is 1.38 kj/g
Answers: 3
You know the right answer?
The following data were obtained for the reaction 2A + B → C where rate = Δ[C]/Δt [A](M) [B](M) Init...
Questions


Computers and Technology, 06.05.2020 20:10








Mathematics, 06.05.2020 20:10

Mathematics, 06.05.2020 20:10

English, 06.05.2020 20:10


Mathematics, 06.05.2020 20:10

Mathematics, 06.05.2020 20:10

History, 06.05.2020 20:10




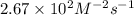
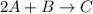
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0588/0800/10aeb.png)
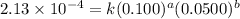 ....(1)
....(1)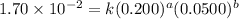 ....(2)
....(2)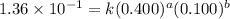 ....(3)
....(3)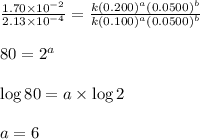
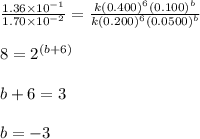
![\text{Rate}=k[A]^6[B]^{-3}](/tpl/images/0588/0800/5c165.png)