
9. Diiodine pentoxide is useful in devices such as respirators because it reacts with the dangerous gas carbon monoxide, CO, to produce relatively harmless CO2 according to the following equation: I2O5 5CO £ I2 5CO2 a. In testing a respirator, 2.00 g of carbon monoxide gas is passed through diiodine pentoxide. Upon analyzing the results, it is found that 3.17 g of I2 was produced. Calculate the percentage yield of the reaction. b. Assuming that the yield in (a) resulted because some of the CO did not react, calculate the mass of CO that passed through.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3


Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
9. Diiodine pentoxide is useful in devices such as respirators because it reacts with the dangerous...
Questions



History, 16.06.2021 22:50

English, 16.06.2021 22:50


Law, 16.06.2021 22:50

Mathematics, 16.06.2021 22:50

Biology, 16.06.2021 22:50



Mathematics, 16.06.2021 22:50

Chemistry, 16.06.2021 22:50

Mathematics, 16.06.2021 22:50




Biology, 16.06.2021 22:50


Mathematics, 16.06.2021 22:50

Mathematics, 16.06.2021 22:50

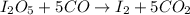
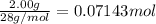
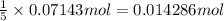 of iodine gas
of iodine gas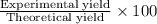
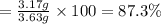
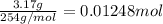
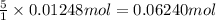 of carbon monoxide
of carbon monoxide

