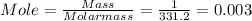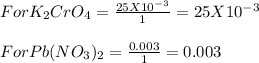4. When aqueous solutions of lead(II) ion are treated with potassium chromate solution, a bright yellow precipitate of lead(II) chromate, PbCrO4, forms. How many grams o lead chromate form when a 1.00-g sample of Pb(NO3)2 is added to 25.0mL of 1.00M K2CrO4 solution

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
4. When aqueous solutions of lead(II) ion are treated with potassium chromate solution, a bright yel...
Questions

Mathematics, 11.03.2021 01:10




Mathematics, 11.03.2021 01:10

Mathematics, 11.03.2021 01:10


Computers and Technology, 11.03.2021 01:10

History, 11.03.2021 01:10

Mathematics, 11.03.2021 01:10

History, 11.03.2021 01:10

Physics, 11.03.2021 01:10


Mathematics, 11.03.2021 01:10


Biology, 11.03.2021 01:10




Mathematics, 11.03.2021 01:10