Chemistry, 08.04.2020 01:15 brianna8739
Commercial cold packs consist of solid ammonium nitrate and water. NH 4NO 3( s) absorbs 25.69 kJ of heat per mole dissolved in water. In a coffee-cup calorimeter, 3.40 g NH 4NO 3( s) is dissolved in 100.0 g of water at 21.0 °C. What is the final temperature of the solution? Assume that the solution has a specific heat capacity of 4.18 J/g•K.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
Commercial cold packs consist of solid ammonium nitrate and water. NH 4NO 3( s) absorbs 25.69 kJ of...
Questions

Mathematics, 28.02.2021 22:20


Biology, 28.02.2021 22:20


Mathematics, 28.02.2021 22:20

Mathematics, 28.02.2021 22:20

Mathematics, 28.02.2021 22:20


Mathematics, 28.02.2021 22:20





Mathematics, 28.02.2021 22:30



Physics, 28.02.2021 22:30

Mathematics, 28.02.2021 22:30

Biology, 28.02.2021 22:30

Business, 28.02.2021 22:30

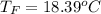
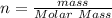
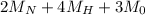
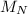 (molar mass of Nitrogen) , 1 for
(molar mass of Nitrogen) , 1 for 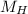 molar mass of hydrogen, 16 for
molar mass of hydrogen, 16 for 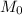 molar mass of oxygen
molar mass of oxygen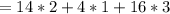
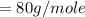
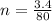
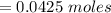

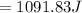
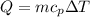
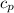 (Specific heat capacity)
(Specific heat capacity) the subject
the subject 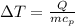
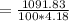
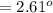 C
C