
Chemistry, 08.04.2020 01:33 imeldachavez124
He thermite reaction, in which powdered aluminum reacts with iron(III) oxide, is highly exothermic. 2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(s) Use standard enthalpies of formation to find for the thermite reaction

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2


Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1
You know the right answer?
He thermite reaction, in which powdered aluminum reacts with iron(III) oxide, is highly exothermic....
Questions

Spanish, 14.01.2022 20:30

Social Studies, 14.01.2022 20:30

Mathematics, 14.01.2022 20:30


Mathematics, 14.01.2022 20:30



Mathematics, 14.01.2022 20:30



Mathematics, 14.01.2022 20:30

Mathematics, 14.01.2022 20:30







Chemistry, 14.01.2022 20:40

Mathematics, 14.01.2022 20:40

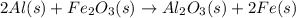 . Use standard enthalpies of formation to find ΔH∘rxn for the thermite reaction. Express the heat of the reaction in kilojoules to four significant figures.
. Use standard enthalpies of formation to find ΔH∘rxn for the thermite reaction. Express the heat of the reaction in kilojoules to four significant figures.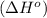 .
.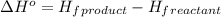
![\Delta H^o=[n_{Fe}\times \Delta H_f^0_{(Fe)}+n_{Al_2O_3}\times \Delta H_f^0_{(Al_2O_3)}]-[n_{Al}\times \Delta H_f^0_(Al)+n_{Fe_2O_3}\times \Delta H_f^0_{(Fe_2O_3)}]](/tpl/images/0588/4390/ac2a9.png)
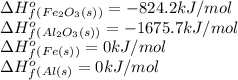
![\Delta H^o_{rxn}=[(2\times 0)+(1\times -1675.5)]-[(2\times 0)+(1\times -824.2)]=-851.5kJ](/tpl/images/0588/4390/65523.png)


