Chemistry, 08.04.2020 01:44 jayline2003
: Citric acid, H3C6H5O7, is a triprotic acid. Consider a buffer system comprising H2C6H5O7 - and HC6H5O7 2- ions. What is the net ionic equation for the reaction that occurs when NaOH is added to a buffer containing H2C6H5O7 - and HC6H5O7 2- ?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1

Chemistry, 23.06.2019 12:50
What is the relative mass of an electron? a) 1/1840 the mass of a neutron + proton b) 1/1840 the mass of an alpha particle c) 1/1840 the mass of a c-12 atom d) 1/1840 the mass of a hydrogen atom
Answers: 3
You know the right answer?
: Citric acid, H3C6H5O7, is a triprotic acid. Consider a buffer system comprising H2C6H5O7 - and HC6...
Questions





Mathematics, 02.11.2020 14:00

Geography, 02.11.2020 14:00


Biology, 02.11.2020 14:00


Mathematics, 02.11.2020 14:00

English, 02.11.2020 14:00

Mathematics, 02.11.2020 14:00

Mathematics, 02.11.2020 14:00

English, 02.11.2020 14:00


English, 02.11.2020 14:00


Physics, 02.11.2020 14:00

English, 02.11.2020 14:10



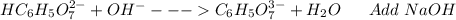
 and
and