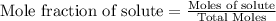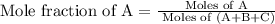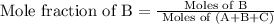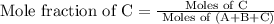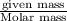Chemistry, 08.04.2020 01:46 bonnysvalentine
A vessel contains a mixture of gases. The mass of each gas used to make the mixture is known. Which of the following information is needed to determine the mole fraction of each gas in the mixture? The molar mass of each gas A The density of the gases in the vessel B The total pressure of the gases in the vessel C The number of atoms per molecule for each gas

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
A vessel contains a mixture of gases. The mass of each gas used to make the mixture is known. Which...
Questions

Mathematics, 10.07.2019 22:00

Mathematics, 10.07.2019 22:00


Mathematics, 10.07.2019 22:00


History, 10.07.2019 22:00

Chemistry, 10.07.2019 22:00




Mathematics, 10.07.2019 22:00


Physics, 10.07.2019 22:00




History, 10.07.2019 22:00

Biology, 10.07.2019 22:00

Mathematics, 10.07.2019 22:00