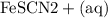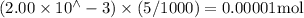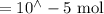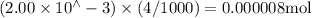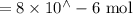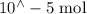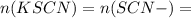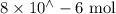Suppose a student mixes 4.00 mL of 2.00 # 10-3 M Fe(NO3 ) 3 with 5.00 mL of 2.00 # 10-3 M KSCN and 1.00 mL of water. The student then determines the [FeNCS2+] at equilibrium to be 8.75 # 10-5 M. Find the equilibrium constant for the following reaction. Show all your calculations for each step. Fe3+ (aq) + SCN- (aq) FeNCS2+ (aq) Step 1. Calculate the initial number of moles of Fe3+ and SCN- (use Equation 12). moles of Fe3+ moles of SCN- Step 2. How many moles of FeNCS2+ are present at equilibrium? What is the volume of the equilibrium mixture? mL moles of FeNCS2+ How many moles of Fe3+ and SCN- are consumed to produce the FeNCS2+? moles of Fe3+ moles of SCN-

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

You know the right answer?
Suppose a student mixes 4.00 mL of 2.00 # 10-3 M Fe(NO3 ) 3 with 5.00 mL of 2.00 # 10-3 M KSCN and 1...
Questions



Mathematics, 12.10.2020 14:01


Law, 12.10.2020 14:01


Mathematics, 12.10.2020 14:01

Mathematics, 12.10.2020 14:01

Mathematics, 12.10.2020 14:01


Mathematics, 12.10.2020 14:01

Chemistry, 12.10.2020 14:01

English, 12.10.2020 14:01



History, 12.10.2020 14:01

Chemistry, 12.10.2020 14:01

Chemistry, 12.10.2020 14:01


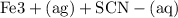 <---->
<---->