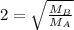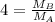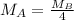At a given temperature and pressure, a sample of Gas A is observed to diffuse twice as fast as a sample of a different gas, B. Based on this: a. The molar mass of A is one fourth that of B b. The molar mass of A is one half that of B c. The molar mass of A is four times that of B d. The molar mass of A is 1.414 times that of B e. The molar mass of A is 0.707 times that of B

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1


Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
At a given temperature and pressure, a sample of Gas A is observed to diffuse twice as fast as a sam...
Questions


Mathematics, 17.10.2021 22:40

Mathematics, 17.10.2021 22:40




Mathematics, 17.10.2021 22:50

English, 17.10.2021 22:50

Social Studies, 17.10.2021 22:50

Mathematics, 17.10.2021 22:50

English, 17.10.2021 22:50

Mathematics, 17.10.2021 22:50


Mathematics, 17.10.2021 22:50




Mathematics, 17.10.2021 22:50

Chemistry, 17.10.2021 22:50

Mathematics, 17.10.2021 22:50

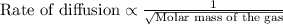
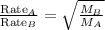
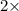 Rate of diffusion of B
Rate of diffusion of B