
Chegg Aqueous hydrobromic acid will react with solid sodium hydroxide to produce aqueous sodium bromide and liquid water . Suppose 17.0 g of hydrobromic acid is mixed with 14. g of sodium hydroxide. Calculate the minimum mass of hydrobromic acid that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
You know the right answer?
Chegg Aqueous hydrobromic acid will react with solid sodium hydroxide to produce aqueous sodium brom...
Questions

Biology, 23.12.2019 19:31

Mathematics, 23.12.2019 19:31

History, 23.12.2019 19:31

Social Studies, 23.12.2019 19:31


Mathematics, 23.12.2019 19:31


Mathematics, 23.12.2019 19:31

Mathematics, 23.12.2019 19:31

History, 23.12.2019 19:31



Mathematics, 23.12.2019 19:31

Mathematics, 23.12.2019 19:31




World Languages, 23.12.2019 19:31


Mathematics, 23.12.2019 19:31

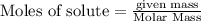
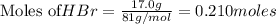
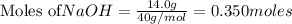

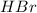 require = 1 mole of
require = 1 mole of 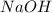
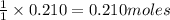 of
of 

