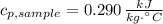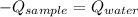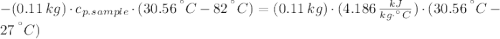Chemistry, 08.04.2020 02:09 lollipop83
A 110.0-g sample of metal at 82.00°C is added to 110.0 g of H O(l) at 27.00°C in an insulated container. The temperature rises to 30.56°C. Neglecting the heat capacity of the container, what is the specific heat of the metal? The specific heat of H O(l) is 4.18 J/(g ∙ °C).

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
A 110.0-g sample of metal at 82.00°C is added to 110.0 g of H O(l) at 27.00°C in an insulated contai...
Questions

Mathematics, 31.01.2020 16:01

Mathematics, 31.01.2020 16:01


History, 31.01.2020 16:01

History, 31.01.2020 16:01

Mathematics, 31.01.2020 16:01

Mathematics, 31.01.2020 16:01






Arts, 31.01.2020 16:02

Mathematics, 31.01.2020 16:02


Social Studies, 31.01.2020 16:02

Biology, 31.01.2020 16:02


History, 31.01.2020 16:02