
C6H12O6 (s) + NH3 (g) -> C3H8O3 (l) + C2H6O(l) +CH1.83O0.56N0.17 (s) + CO2(g) + H2O Note that 1 mole glucose produces 0.5 mol glycerol and that the ratio of NH3 to H20 is 1:1. During preparation , you accidentally add too much glucose. However, you decide to let the bioreactor run anyway. After the reaction goes to completion at least one of the starting reactants is used up, you discover that 1.4 lb-m ethanol had been produced. How much heat had been produced and subsequently removed from the system. How much glucose did you initially add to the reactor?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3
You know the right answer?
C6H12O6 (s) + NH3 (g) -> C3H8O3 (l) + C2H6O(l) +CH1.83O0.56N0.17 (s) + CO2(g) + H2O Note that 1 m...
Questions

Mathematics, 22.06.2019 02:00

Mathematics, 22.06.2019 02:00




Arts, 22.06.2019 02:00

Chemistry, 22.06.2019 02:00

Mathematics, 22.06.2019 02:00


Mathematics, 22.06.2019 02:00


Mathematics, 22.06.2019 02:00





English, 22.06.2019 02:00


English, 22.06.2019 02:00

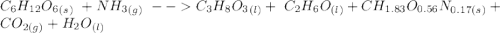
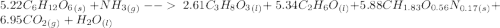
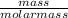
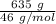
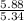 moles of S. cerevisiae formed.
moles of S. cerevisiae formed.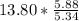 moles of S. cerevisiae formed
moles of S. cerevisiae formed

