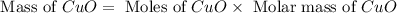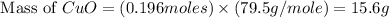Chemistry, 08.04.2020 02:26 Perdikaris
. In the production of copper from ore containing copper(II) sulfide, the ore is first roasted to change it to the oxide according to the following equation: 2CuS 3O2 £ 2CuO 2SO2 a. If 100 g of CuS and 56 g of O2 are available, which reactant is limiting? b. What mass of CuO can be formed from the reaction of 18.7 g of CuS and 12.0 g of O2? 4. A reaction such as

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

You know the right answer?
. In the production of copper from ore containing copper(II) sulfide, the ore is first roasted to ch...
Questions

Mathematics, 02.08.2021 21:50


Mathematics, 02.08.2021 21:50

Mathematics, 02.08.2021 21:50



Mathematics, 02.08.2021 21:50

Mathematics, 02.08.2021 21:50




Geography, 02.08.2021 21:50



Biology, 02.08.2021 21:50


Mathematics, 02.08.2021 21:50

Biology, 02.08.2021 21:50


Mathematics, 02.08.2021 21:50

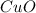 produced is, 15.6 grams.
produced is, 15.6 grams. = 100 g
= 100 g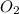 = 56 g
= 56 g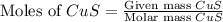
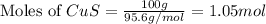
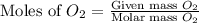
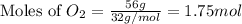
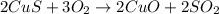
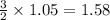 moles of
moles of 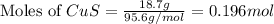
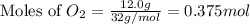
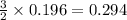 moles of
moles of