
Chemistry, 08.04.2020 02:35 brianadee800
In the reaction of nitrogen gas, N2, with hydrogen gas, H2, to form ammonia gas, NH3, how many moles of hydrogen are needed to react with two moles of nitrogen? N2 + 3H2 → 2NH3 Select one: A. 5.710 moles B. 4 moles C. 2 moles D. 8 moles E. 6 moles

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 09:50
When scientists are ready to publish the results of their experimentation, why is it important for them to include a description of the procedures they used?
Answers: 1

Chemistry, 23.06.2019 10:30
Which of the following pairs of elements is most likely to form an ionic compound? a oxygen and fluorine b sodium and aluminum c calcium and chlorine d nitrogen and sulfur
Answers: 1
You know the right answer?
In the reaction of nitrogen gas, N2, with hydrogen gas, H2, to form ammonia gas, NH3, how many moles...
Questions

World Languages, 15.12.2020 23:10


Biology, 15.12.2020 23:10


Mathematics, 15.12.2020 23:10



Mathematics, 15.12.2020 23:10

Chemistry, 15.12.2020 23:10


Mathematics, 15.12.2020 23:10


History, 15.12.2020 23:10




Mathematics, 15.12.2020 23:10


Mathematics, 15.12.2020 23:10

Arts, 15.12.2020 23:10

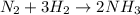
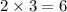 moles of hydrogen gas
moles of hydrogen gas

