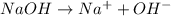Chemistry, 08.04.2020 02:33 cmflores3245
An Arrhenius base is best defined as a. An Arrhenius base is best defined as a b. hydroxide acceptor. c. proton donor. d. substance that dissociates in water to produce aqueous hydrogen ions.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3

Chemistry, 23.06.2019 15:20
Which element below could be an isotope of berylliumsodium-10beryllium-10boron-9carbon-9
Answers: 2
You know the right answer?
An Arrhenius base is best defined as a. An Arrhenius base is best defined as a b. hydroxide acceptor...
Questions

SAT, 15.12.2020 04:00

Mathematics, 15.12.2020 04:00


Mathematics, 15.12.2020 04:00


Physics, 15.12.2020 04:00


History, 15.12.2020 04:00


Health, 15.12.2020 04:00


Mathematics, 15.12.2020 04:00




Mathematics, 15.12.2020 04:00

Mathematics, 15.12.2020 04:00



Mathematics, 15.12.2020 04:00

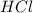 is an Arrhenius acid.
is an Arrhenius acid.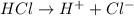
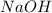 is an Arrhenius base.
is an Arrhenius base.