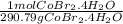Chemistry, 08.04.2020 02:56 sairaanwar67
Calculate the molarity of a solution obtained dissolving 10.0 g of cobalt(Ⅱ) bromide tetrahydrate in enough water to make 450 mL of solution
a. 6.80 × 10-2
b. 7.64 × 10-2
c. 7.64 × 10-5
d. 7.51 × 10-2
e. 0.102 M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1


Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Calculate the molarity of a solution obtained dissolving 10.0 g of cobalt(Ⅱ) bromide tetrahydrate in...
Questions


Mathematics, 12.10.2019 08:10

Mathematics, 12.10.2019 08:10


Mathematics, 12.10.2019 08:10


Chemistry, 12.10.2019 08:10

Mathematics, 12.10.2019 08:10

Mathematics, 12.10.2019 08:10

Geography, 12.10.2019 08:10

Mathematics, 12.10.2019 08:10


Computers and Technology, 12.10.2019 08:10







Mathematics, 12.10.2019 08:10