

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Stock solutions of nitric acid have concentration of 16.0 M. What volume of stock solution is needed...
Questions



Mathematics, 12.02.2022 21:50


History, 12.02.2022 21:50

English, 12.02.2022 21:50


Mathematics, 12.02.2022 21:50



Mathematics, 12.02.2022 21:50



Mathematics, 12.02.2022 21:50


Mathematics, 12.02.2022 21:50

Mathematics, 12.02.2022 22:00


Mathematics, 12.02.2022 22:00

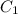 = 16 M
= 16 M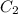 = 2 M
= 2 M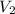 = 2 L = 2000 mL
= 2 L = 2000 mL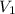
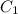 x
x 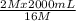 ⇒
⇒ 


