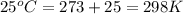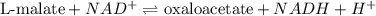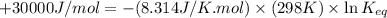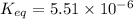Chemistry, 08.04.2020 03:26 pinkmoonlight
In the citric acid cycle, malate is dehydrogenated to oxaloacetate in a highly endergonic reaction with a ΔG’o of +30 kJ mol-1: L‐malate + NAD+ ⇌ oxaloacetate + NADH + H+ Calculate the equilibrium constant K’eq of this reaction. What is the implication of this result?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1
You know the right answer?
In the citric acid cycle, malate is dehydrogenated to oxaloacetate in a highly endergonic reaction w...
Questions

Spanish, 16.11.2019 07:31

Social Studies, 16.11.2019 07:31



History, 16.11.2019 07:31

Mathematics, 16.11.2019 07:31


Mathematics, 16.11.2019 07:31

Mathematics, 16.11.2019 07:31

Mathematics, 16.11.2019 07:31








Social Studies, 16.11.2019 07:31

English, 16.11.2019 07:31

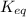 of this reaction is,
of this reaction is, 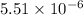
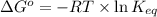
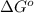 = standard Gibbs free energy = +30 kJ/mol = +30000 J/mol
= standard Gibbs free energy = +30 kJ/mol = +30000 J/mol