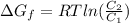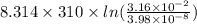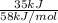Gastric juice (pH 1.5) is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. Calculate the amount of free energy required to concentrate the H in 1 liter of gastric juice at 37 degree of centigrade. Under cellular conditions, how many moles of ATP must be hydrolyzed to provide this amount of free energy

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
Gastric juice (pH 1.5) is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. Calcu...
Questions

Biology, 21.06.2019 20:50

Mathematics, 21.06.2019 20:50



Biology, 21.06.2019 20:50

Geography, 21.06.2019 20:50






History, 21.06.2019 20:50




Business, 21.06.2019 20:50

Chemistry, 21.06.2019 20:50



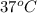 = (37 + 273) K
= (37 + 273) K![-log [H^{+}]](/tpl/images/0588/8520/822be.png)
![[H^{+}]](/tpl/images/0588/8520/85507.png) as follows.
as follows.![[H^{+}] = 10^{-pH}](/tpl/images/0588/8520/241df.png)
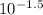
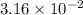 M (
M (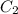 )
)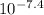
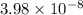 M (
M (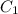 )
)