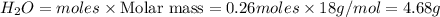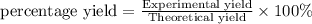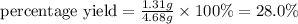Chemistry, 08.04.2020 04:49 creepycrepes
Gaseous butane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . If of water is produced from the reaction of of butane and of oxygen gas, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1
You know the right answer?
Gaseous butane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water ....
Questions

Mathematics, 27.05.2021 23:20

Mathematics, 27.05.2021 23:20

History, 27.05.2021 23:20





History, 27.05.2021 23:20



Mathematics, 27.05.2021 23:20

Mathematics, 27.05.2021 23:20

Mathematics, 27.05.2021 23:20


History, 27.05.2021 23:20

Mathematics, 27.05.2021 23:30


History, 27.05.2021 23:30

Mathematics, 27.05.2021 23:30

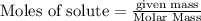
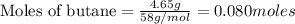
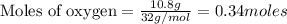
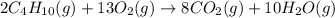
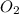 require 2 moles of butane
require 2 moles of butane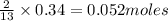 of butane
of butane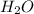
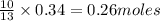 of
of