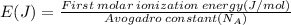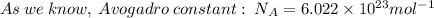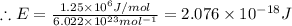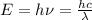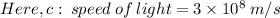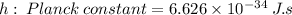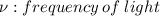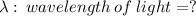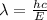Chemistry, 08.04.2020 04:23 quinnbee23
The first ionization energy of chlorine is 1.25x103 kJ/mol. What is the wavelength of light in units of meters (absorbed) which can ionize a chlorine atom? A. 9.57x10–8 B. 3.13x1015 C. 1.89x1036 D. 3.13x1012 E. 9.57x10–5

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

You know the right answer?
The first ionization energy of chlorine is 1.25x103 kJ/mol. What is the wavelength of light in units...
Questions

History, 05.05.2020 06:30

Computers and Technology, 05.05.2020 06:30

History, 05.05.2020 06:30

Mathematics, 05.05.2020 06:30

Mathematics, 05.05.2020 06:30

Mathematics, 05.05.2020 06:30

Mathematics, 05.05.2020 06:30

Biology, 05.05.2020 06:30

English, 05.05.2020 06:30

Mathematics, 05.05.2020 06:30






Mathematics, 05.05.2020 06:30