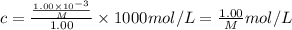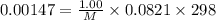A chemist is trying to determine the molar mass of a certain protein. 1.00 x 10 -3 g of it was dissolved in enough water to make 1.00 mL of solution. The osmotic pressure of this solution was found to be 1.12 torr at 25.0°C. Calculate the molar mass of the protein.

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2


Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
A chemist is trying to determine the molar mass of a certain protein. 1.00 x 10 -3 g of it was disso...
Questions


Mathematics, 10.02.2021 18:10




Social Studies, 10.02.2021 18:10

Mathematics, 10.02.2021 18:10


English, 10.02.2021 18:10

Mathematics, 10.02.2021 18:10

English, 10.02.2021 18:10

Advanced Placement (AP), 10.02.2021 18:10




Mathematics, 10.02.2021 18:10



Mathematics, 10.02.2021 18:10

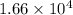 g/mol
g/mol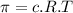
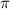 represents osmotic pressure of solution, c represents molarity of solution and T represents temperature in kelvin scale.
represents osmotic pressure of solution, c represents molarity of solution and T represents temperature in kelvin scale.