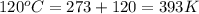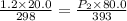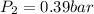Chemistry, 08.04.2020 04:33 guillmar003
A gas inside a piston initially has a pressure of 1.2 bars at temperature of 25 degree C and volume of 20.0 mL. If temperature is increased to 120 deg C and the piston expands to a volume of 80 mL, what is the new pressure

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
A gas inside a piston initially has a pressure of 1.2 bars at temperature of 25 degree C and volume...
Questions







English, 19.05.2021 06:10

Mathematics, 19.05.2021 06:10

Mathematics, 19.05.2021 06:10

English, 19.05.2021 06:10


Biology, 19.05.2021 06:10

Mathematics, 19.05.2021 06:10

Spanish, 19.05.2021 06:10

Mathematics, 19.05.2021 06:10

Spanish, 19.05.2021 06:10

Mathematics, 19.05.2021 06:10


Spanish, 19.05.2021 06:10

English, 19.05.2021 06:10

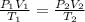
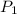 = initial pressure of gas = 1.2 bar
= initial pressure of gas = 1.2 bar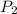 = final pressure of gas = ?
= final pressure of gas = ?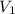 = initial volume of gas = 20.0 ml
= initial volume of gas = 20.0 ml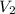 = final volume of gas = 80.0 ml
= final volume of gas = 80.0 ml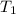 = initial temperature of gas =
= initial temperature of gas = 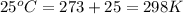
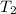 = final temperature of gas =
= final temperature of gas =