Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1


Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
Sodium hydroxide reacts with aluminum and water to produce hydrogen gas:2 Al(s) + 2 NaOH(aq) + 6 H2O...
Questions






History, 25.07.2019 04:50

History, 25.07.2019 04:50




Physics, 25.07.2019 04:50

Computers and Technology, 25.07.2019 04:50








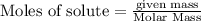
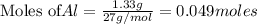
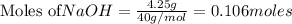
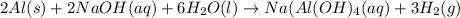
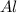 require = 2 moles of
require = 2 moles of ![NaOH/tex]Thus 0.049 moles of [tex]Al](/tpl/images/0589/0248/32375.png) will require=
will require=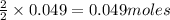 of
of 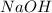
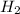
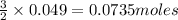 of
of