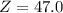Chemistry, 08.04.2020 05:03 munozjosue258
Chromium has four naturally occurring isotopes. Chromium-50 has a percent abundance of 4.35%, chromium-52 has a percent abundance of 83.79%, chromium-53 has a percent abundance of 9.50%, and chromium-54 has a percent abundance of 2.37%. Based on this information calculate the average atomic mass of chromium. *

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1

You know the right answer?
Chromium has four naturally occurring isotopes. Chromium-50 has a percent abundance of 4.35%, chromi...
Questions

Biology, 10.03.2021 20:30




Mathematics, 10.03.2021 20:30

History, 10.03.2021 20:30


English, 10.03.2021 20:30


History, 10.03.2021 20:30

Chemistry, 10.03.2021 20:30





Mathematics, 10.03.2021 20:30

Health, 10.03.2021 20:30


Mathematics, 10.03.2021 20:30

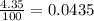
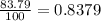
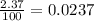
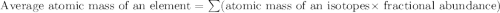
![Z=[(50\times 0.0435)+(52\times 0.8379)+(54\times 0.0237)]](/tpl/images/0589/0146/846f5.png)