

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:10
Seawater contains approximately 3.5%nacl by mass and has a density of 1.02 g/ml. what volume of seawater contains 7.5 g of sodium?
Answers: 2

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
C3H8+5O2 -> 3CO2+4H2O How many moles of CO2 will be produced from 97.0 g of C3H8, assuming O2 is...
Questions



Physics, 11.06.2021 14:00

Social Studies, 11.06.2021 14:00

History, 11.06.2021 14:00

Mathematics, 11.06.2021 14:00

Mathematics, 11.06.2021 14:00

Mathematics, 11.06.2021 14:00

Social Studies, 11.06.2021 14:00


History, 11.06.2021 14:00

Social Studies, 11.06.2021 14:00


Social Studies, 11.06.2021 14:00

Chemistry, 11.06.2021 14:00


Mathematics, 11.06.2021 14:00


Mathematics, 11.06.2021 14:00

Mathematics, 11.06.2021 14:00

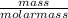
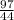 = 2.21moles
= 2.21moles 

