
Chemistry, 08.04.2020 17:26 arielmajesty9107
1. 2Mg + O₂ → 2MgO How many moles of MgO form if 6mol of oxygen react? *
2. 2Mg + O₂ → 2MgO How many moles of oxygen are required to react with 3.6mol of magnesium?
3. 2Mg + O₂ → 2MgO How many moles of magnesium are required to produce 5.5mol of MgO?

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
1. 2Mg + O₂ → 2MgO How many moles of MgO form if 6mol of oxygen react? *
2. 2Mg + O₂ → 2MgO Ho...
2. 2Mg + O₂ → 2MgO Ho...
Questions

Mathematics, 31.03.2021 02:00



Mathematics, 31.03.2021 02:00



Biology, 31.03.2021 02:00

Geography, 31.03.2021 02:00


English, 31.03.2021 02:00

Computers and Technology, 31.03.2021 02:00

Mathematics, 31.03.2021 02:00

Mathematics, 31.03.2021 02:00

History, 31.03.2021 02:00

Mathematics, 31.03.2021 02:00


History, 31.03.2021 02:00

History, 31.03.2021 02:00


Physics, 31.03.2021 02:00

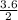 = 1.8 moles of O₂
= 1.8 moles of O₂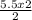 = 5.5 moles of Mg
= 5.5 moles of Mg

