Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
You know the right answer?
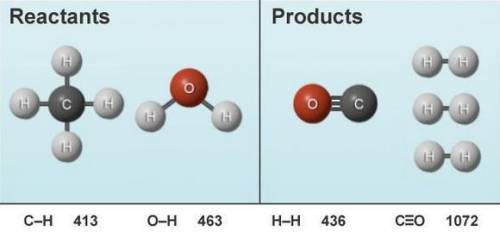
The following reactant molecules are rearranged to form the product molecules shown. The relevant bo...
Questions


Social Studies, 23.11.2020 03:20



English, 23.11.2020 03:20

Business, 23.11.2020 03:20

Mathematics, 23.11.2020 03:20


Mathematics, 23.11.2020 03:20

Mathematics, 23.11.2020 03:20

English, 23.11.2020 03:20




Mathematics, 23.11.2020 03:20