Given the reaction represented by the balanced equation:
CH4(g)+3F2(g)->3HF(g)+CHF3 c...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2

Chemistry, 23.06.2019 22:30
The reversible reaction for ph indicator bromophenol blue is (c19h8br4o5s)h- < --> (c19h8br4o5s)2-+h+ write the equilibrium expression for this reaction
Answers: 3
You know the right answer?
Questions

Biology, 08.10.2019 23:30

Health, 08.10.2019 23:30

Social Studies, 08.10.2019 23:30

Health, 08.10.2019 23:30


History, 08.10.2019 23:30



Mathematics, 08.10.2019 23:30


History, 08.10.2019 23:30

Mathematics, 08.10.2019 23:30

Geography, 08.10.2019 23:30

History, 08.10.2019 23:30


English, 08.10.2019 23:30

Computers and Technology, 08.10.2019 23:30


Health, 08.10.2019 23:30

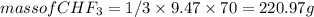
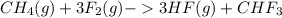
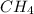 ) is taken excess amount
) is taken excess amount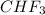 depend only on mass of fluorine
depend only on mass of fluorine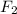 =180 g
=180 g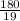 =9.47
=9.47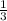 mole of
mole of 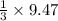 mole of
mole of 

